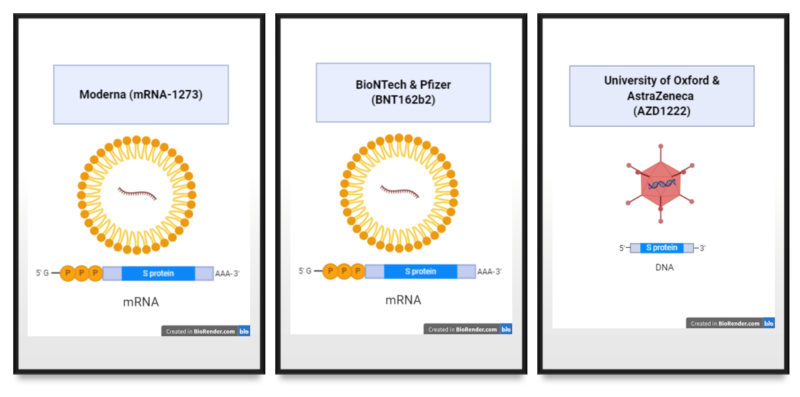
The excellent news is that now we have 3 vaccines that seem to have good efficacy. Which means they shield the individuals who obtain the vaccine. Two of the vaccines are primarily based a brand new expertise that has not been used earlier than in vaccines. Though the expertise is new, it isn’t fully uncommon in that it depends on offering directions (within the type of nucleic acid) in your personal cells to make a protein that the virus makes. Different current vaccine applied sciences do the identical factor, they only present the directions in a distinct type of nucleic acid and delivered in a distinct form of “bundle” (Determine 1).
Pfizer and BioNTech reported that their vaccine was 95% efficient at stopping COVID-19 starting 28 days after the primary dose. They enrolled greater than 43,000 individuals within the medical trial and noticed 170 instances of COVID-19. Of these 170 instances, solely 8 have been within the group that had obtained the vaccine. That is how they arrived on the 95% efficient worth. There have been 10 extreme instances of COVID-19 and 9 of these have been the placebo group. Moreover, the vaccine was 94% efficient in adults over 65 years previous. This info is all coming from a press launch, so the outcomes haven’t be printed and peer reviewed but. The outcomes are optimistic sufficient that the businesses have utilized for emergency use authorization from the FDA.
Limitations are that this vaccine requires 2 doses to attain the reported efficacy and requires storage and transportation at an especially low temperature, -70°C (-94°F) to keep up stability. That is excellent news however not “save the world” information, due to the storage and transportation necessities.
Moderna additionally reported that their vaccine was 94% efficient primarily based on 95 instances of COVID-19 within the greater than 30,000 contributors within the medical trial. Among the many 95 instances, solely 5 have been within the group that obtained the vaccine. The entire instances of extreme COVID-19 (11 instances) have been within the placebo group. Just like the Pfizer and BioNTech vaccine outcomes, these outcomes are coming from a press launch. The European Medicines Company is performing a evaluation of this vaccine as a part of the following step to vaccine approval in Europe.
Limitations are that this vaccine requires 2 doses to attain the reported efficacy and the vaccine requires a better quantity of fabric than the Pfizer and BioNTech vaccine. Nevertheless, this vaccine is steady at steady at -20°C (-4°F) for as much as six months, at refrigerated circumstances for as much as 30 days, and at room temperature for as much as 12 hours. That is nearer to “save the world” information.
These 2 vaccines use mRNA, a kind of directions that cells use to supply proteins. Utilizing the mRNA molecules, cells injected with the vaccine make a protein from the SARS-CoV-2 virus referred to as Spike or S. The S protein is required for the virus to enter into cells. So an immune response mounted in opposition to the S protein has a excessive likelihood of producing immunity that stops an infection. This speculation appears to be true primarily based on the outcomes of the medical trials with the mRNA-based vaccines.
AstraZeneca additionally reported optimistic outcomes with their vaccine. Their vaccine additionally supplies cells directions for making the S protein from SARS-CoV-2. Nevertheless, the directions are packaged inside an engineered virus referred to as chimpanzee adenovirus. This adenovirus can not replicate and as an alternative is used as a supply system for the directions for making S. The benefit to this kind of vaccine is it’s a DNA vaccine delivered inside a virus particle, which is extra steady than an mRNA vaccine delivered inside a lipid nanoparticle (a tiny soap-like bubble) (Determine 1).
AstraZeneca reported outcomes from a research with 23,000 contributors who have been in one of many following teams: One group obtained 2 doses of a half dose adopted by a full dose, a second obtained 2 doses of the full-dose quantity, and the final obtained a vaccine in opposition to a distinct pathogen adopted by an injection of saline. The completely different dosing regimens had completely different efficacies: the half dose adopted by a full dose had 90% efficacy and the complete dose adopted by one other full dose had 62% efficacy. Thus, many information experiences have said the common of 70%. On condition that the half-dose/full-dose routine had a greater impact and that much less materials could be required, it appears unlikely that AstraZeneca would proceed with the two full-dose routine. Nevertheless, it’s doable that completely different teams, like older individuals, might reply in another way and profit extra from the upper dosing routine.
Among the many issues we have no idea but are if both of those 3 vaccines stop asymptomatic illness or unfold of the virus. We additionally have no idea how lengthy immunity will final. What we do know is that the vaccines which can be in improvement seem to work and that with combos of varied vaccines, we are going to attain “save the world” information.
Press Releases
Medical trials
Science Articles
Extra Individuals Are Getting COVID-19 Twice, Suggesting Immunity Wanes Shortly in Some
‘Simply stunning’: One other COVID-19 vaccine, from newcomer Moderna, succeeds in large-scale trial
Cite as: N. R. Gough, Good Information on the COVID-19 Entrance. BioSerendipity (23 November 2020) https://www.bioserendipity.com/good-news-on-the-covid-19-front/.














